Posts Tagged ‘recall’
Defective Bikes and Equipment List for Cyclists
 Each spring, cyclists throughout Massachusetts say goodbye to winter and put their bikes back on the road. But whether you cycle every day or just occasionally, now is the time to make sure your bike and equipment meets the latest safety regulations.
Each spring, cyclists throughout Massachusetts say goodbye to winter and put their bikes back on the road. But whether you cycle every day or just occasionally, now is the time to make sure your bike and equipment meets the latest safety regulations.
It is good practice to test and inspect key parts of your bike, such as the quick release wheels, brakes and pedals.Then check with the manufacturer of your bicycle. Look online and see if if offers an owners’ manual. If you have not done so, register your bike so you may receive recall notices.
You can also check the Consumer Product Safety Commission (CPSC) website for recent bicycle recalls. Each year, the CPSC recalls hundreds of thousands of bicycles and parts after receiving reports of defects and injuries.
Here are a few recent bicycle and equipment recalls from the CPSC:
Recalled Bicycles. Some 91,000 Bridgeway Bicycles were recalled in September 2011 because of a defective bicycle chain which can break, causing the rider to lose control and fall. The CPSC received 11 incident reports, including injuries, lacerations and contusions. Read more.
Children Bicycle Seats and Trailers. Two of the largest bicycle-related recalls involve defective children’s equipment. Topeak Babyseat II Bicycle Carrier Seats were recalled in April 2012 after two reports of near amputations and crushed fingers. When a child is lifted out of the seat, their fingers can get caught in a defective hinge mechanism. The product recall affected 30,400 consumers. Read more.
In January 2012, 44,000 Chariot bicycle trailers and 70,000 trailer conversion kits were recalled after 24 incident reports around the world, including three in the U.S. The trailer’s hitch mechanism can crack and break, causing the trailer to detach from the bicycle. Read more.
Helmets. Little Tricky Bicycle Helmets recalled 30,400 bicycle helmets in January 2012. Product testing demonstrated the helmets did not comply with CPSC safety standards for impact resistance. Read more.
Click for a full list of recent bicycle-related recalls from the CPSC.
Read More
Defective Pool Slide Kills Woman in Andover, Mass.
 About 21,000 inflatable swimming pool slides are being recalled after the death of a 29-year-old woman in Massachusetts and two other people sustained serious personal injuries.
About 21,000 inflatable swimming pool slides are being recalled after the death of a 29-year-old woman in Massachusetts and two other people sustained serious personal injuries.
The Consumer Product Safety Commission (CPSC), Walmart of Bentonville, Ark. and Toys R Us Inc., of Wayne, NJ announced the recall Thursday, May 10. The Banzai inflatable water slides are designed for use with in-ground pools, but the CPSC says they pose a risk for injury. They can deflate and a user can hit the cement ground underneath the slide. The slide is also unsecure and can fall over, in both windy and still conditions. Finally, it carries inadequate warnings and instructions for users.
The CPSC is aware of one death and two serious personal injuries. In one case, a 29-year-old Colorado mother died in Andover, to the north of Boston. The woman died after going down a Banzai inflatable slide and hitting her head on the pavement below. The slide had been partially deflated.
The two injuries occurred in a similar manner, one leaving a 24-year-old man from Springfield, Mo. a quadriplegic. In a third case, an Allentown, Pa. woman fractured her neck.
The recalled pool slides were manufactured in China by Manley Toys, Ltd. and sold at Walmart and Toys R Us stores nationwide from January 2005 through June 2009. The defective product was sold for about $250. The vinyl slides have a blue base, yellow sliding mat and an arch going over the slide. The words ‘Banzai Splash’ are printed on the side of the defective slide.
The CPSC urges consumers to immediately stop using the defective product and return it to a Walmart or Toys R Us for a full refund. Consumers can also cut out the two safety warnings on the slide and return those for a refund. For additional information, visit www.walmart.com and www.toysrus.com.
Consumers can determine whether they have the slide by clicking here to look at pictures posted by the CPSC. If you still have the box and packaging, look for barcode number 2675315734 and model number 15734.
Read More
Recalled Birth Control Pills: Lo/Ovral-28, Norgestrel and Ethinyl Estradiol
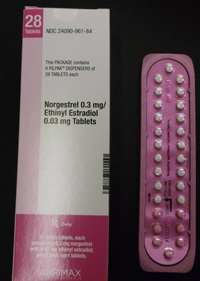 Pfizer Inc. has voluntarily recalled certain lots of birth control pills which may contain ingredient errors or out-of-sequence packaging which could have exposed women to a risk for unintended pregnancy.
Pfizer Inc. has voluntarily recalled certain lots of birth control pills which may contain ingredient errors or out-of-sequence packaging which could have exposed women to a risk for unintended pregnancy.
In January, Pfizer recalled 14 lots of Lo/Ovral-28 (norgestrel and ethinyl estradiol) Tablets and 14 lots of Norgestrel and Ethinyl Estradiol Tablets (generic) for customers in the U.S. market. The defective pills were distributed to warehouses, clinics and retail pharmacies nationwide.
An investigation by Pfizer found that some blister packs may contain an inexact count of inert or active ingredient tablets and that the tablets may be out of sequence. Pfizer recalled the tablets on January 31, 2012, with knowledge of the Food and Drug Administration (FDA). Pfizer said the error has been corrected.
The tablets were manufactured and packaged by Pfizer Inc., commercialized by Akrimax Rx Products and labeled under the Akrimax Pharmaceuticals brand. The medicine is packaged in blister packs of 21 tablets of active ingredients and seven tablets of inert ingredients. Click the link below for packaging numbers involved in the recall.
The product liability lawyers at Breakstone, White & Gluck are reviewing cases for women who have taken defective lots of these birth control pills and have experienced or are experiencing an unplanned pregnancy. Contact us today at 800-3791244 or 617-723-7676 or use our contact form. Read More
Product Recall: More than 10,000 Fuji Bicycles Recalled
 The U.S. Consumer Product Safety Commission announced the recall this week of more than 10,000 defective bicycles after reports the product’s frame was breaking.
The U.S. Consumer Product Safety Commission announced the recall this week of more than 10,000 defective bicycles after reports the product’s frame was breaking.
Fuji Saratoga Women’s Bicycles recalled about 10,500 bicycles sold nationwide from November 2007 through December 2011. The bicycles were recalled after the company became aware of 12 reports of bicycle frames breaking. There were two injuries reported, including a head laceration requiring 20 stitches.
The product’s defect is its frame was breaking in the center of the downtube during use, causing bicyclists to lose control and fall. Bicyclists are instructed to stop riding and seek a replacement bike frame.
About the recalled Fuji women’s cruisers bicycles:
- 2008 to 2010 models of Saratoga 1.0, Saratoga 2.0, Saratoga 3.0 and Saratoga 4.0. The model type will be printed on the bike.
- The bikes come in various colors.
- The bikes will have the words “Fuji” and “Saratoga” alone or “Saratoga” printed on the frame.
- Serial numbers beginning with ICFJ7, ICFJ8, ICFJ9, ICFJ10 and ICFJ11. The serial number is located on the bottom of the frame near the crank.
The defective products were imported by Advanced Sports, Inc. of Philadelphia and manufactured in China. They were sold at specialty bike shops.
Customers are instructed to obtain a free replacement frame. They can contact Advanced Sports Inc. toll-free at 888-286-6263 between 8 a.m. and 4:30 p.m. Monday through Friday or visit www.fujibikes.com. They can also return the bike to any authorized Fuji Bicycle dealer for the free part.
Click here for more information on this recall.
In a smaller recall, the Mountain Bicycle Handlebar Stem has been recalled in the U.S. and Canada. Some 213 units were recalled in the U.S. and 83 in Canada by the importer, Shimano American Corp. of Irvine, Calif. The defective bicycles were sold at REI stores nationwide from October 2009 to November 2010 for about $120.
The bicycles were recalled because the bolt holding the front plate of the stem to the stem body can be pulled out of the threads while the bike is being ridden, causing the rider to fall. There has been one report of a rider falling and sustaining torso and arm injuries. Click here for more information on this recall.
The Boston product liability lawyers at Breakstone, White & Gluck have over 80 years combined experience handling complex cases involving serious personal injuries, wrongful death and defective products. We have obtained clients compensation in cases involving defective motor vehicles, recalled medical devices and dangerous pharmaceuticals.
If you have been injured, it is important to learn your rights and how long you may have to file a claim. For a free legal consultation, contact us today at 800-379-1244 or 617-723-7676 or use our contact form.
Recalled Coffee Makers Burn Nearly 40 People
 More than 1.7 million coffee makers have been recalled after reports some machines have sprayed hot liquid, leaving 37 people with second-degree burns.
More than 1.7 million coffee makers have been recalled after reports some machines have sprayed hot liquid, leaving 37 people with second-degree burns.
The Tassimo Single-Cup Brewers were recalled Feb. 9 by BSH Home Appliances Corp. of Irvine, California. Some 835,000 machines were recalled in the United States and 900,000 were recalled in Canada. The California manufacturer recalled the defective product voluntarily along with the Consumer Product Safety Commission (CPSC) and Health Canada.
The brewers are defective because they can burst and spray hot liquid and coffee grounds or tea leaves onto consumers. There were a total of 140 reports of the brewers spraying hot liquid. Among the 37 second-degree burns was a 10-year-old Minnesota girl who suffered serious facial and neck burns which required her to be hospitalized.
The defective coffee makers carried the brand names of Bosch and Tassimo Professional Brewers. The Bosch brewers were sold in several colors to consumers between the dates of June 2008 and February 2012 for between $100 and $250. The Tassimo Professional was sold on in black, directly to hotels and food service providers. The brewers were manufactured in Slovenia and China.
Consumers are advised to stop using the recalled coffee makers immediately and contact the firm to order a free replacement T Disc holder part to fix the mechanism. This is the part of the machine that holds the single serve coffee cup.
Consumers can visit www.tassimodirect.com/safetyrecall for the full list of recalled models and to request a replacement part. They can also call the firm toll-free at 866-918-8763.
Click here to read the recall notice from the CPSC.
Read More
DePuy Hip Implant Recall Leads Insurers to Seek Recovery
Medical insurance companies are alerting patients who have defective all-metal hip implants that they will seek to recover expenses from any settlement money which patients receive. Medicare is also expected to try and recover its costs.
Thousands of patients have been affected by recent recalls of all-metal hip implants and are expected to seek compensation from manufacturers. Although insurance companies, by statute or contract, often have the right to recover some of their costs, it is unusual for companies to be so assertive for medical device failures. The aggressive actions being taken by the insurers reflect their awareness of just how expensive these defective products are becoming.
In August 2010, DePuy recalled the ASR XL Acetabular System. The hip implant is defective because it causes friction between the metallic ball and socket components, which are implanted to replace the femur and acetabulum.
The recalled DePuy hip implants can wear down and produce a substantial amount of metallic particles in patients’ bloodstreams. Patients reported signs of metallosis, including pain, swelling, problems walking and rashes. Other serious problems may include bone fractures, joint inflammation, hip dislocation and damage to the tissue, hip implant loosening, nerves and muscles near the implant.
DePuy recalled the defective hip implants after the Food and Drug Administration (FDA) received about 400 complaints in two years from patients. That number has grown significantly following the recall. In just the first six months of 2011, the FDA received more than 5,000 reports about problems with all-metal hip implants, according to an analysis by The New York Times. DePuy hip implants accounted for 75 percent of the complaints.
It is still unclear how many patients have been affected by defective metal hip implants. The New York Times reports one estimate that 500,000 patients have received an all-metal replacement hip.
The newspaper reports another estimate that 250,000 hip replacements are performed in the United States each year. Until recently all-metal hip implants accounted for nearly one-third of these procedures.
As of October 2011, 3,500 patients filed lawsuits against DePuy in connection with last year’s recall. DePuy also faces over 560 lawsuits in connection with the Pinnacle, another defective all-metal hip implant.
More lawsuits are expected against DePuy and other manufacturers as patients start experiencing pain and requiring corrective surgery. This procedure can be painful, require substantial post-operation bed rest and treatment and cost hundreds of thousands of dollars.
Click here to read more about all-metal hip implants in the New York Times.
Read More
Preventing Food Poisoning After Ground Beef Recall
 A Northeastern grocery store chain has recalled various packages of ground beef after 14 people have been infected with an antibiotic-resistant strain of Salmonella.
A Northeastern grocery store chain has recalled various packages of ground beef after 14 people have been infected with an antibiotic-resistant strain of Salmonella.
Hannaford, of Scarborough, Maine, issued the voluntary recall Thursday, Dec. 15, for an undetermined amount of fresh ground beef that may be contaminated with Salmonella Typhimurium. This strain of Salmonella is resistant to treatment by many antibiotics, including drug classes such as beta-lactams and aminoglycosides. Seven of the 14 people who suffered food poisoning were hospitalized.
The United States Department of Agriculture (USDA) has classified the recall Class 1 with a high health risk. This classification means there is a reasonable probability that use of a product will cause serious, adverse health consequences or death.
The grocer recalled 10 different types of ground beef, ranging from 73 percent to 90 percent. Hannaford said most of the affected individuals had consumed 85 percent. The affected packages have the sell-by date of Dec. 17, 2011 or earlier. They were sold at Hannaford stores in Massachusetts, Maine, New Hampshire, New York and Vermont. Hannaford is offering consumers a full refund.
The ground beef involved in the food poisoning outbreak was sold under the brand names of Hannaford, Taste of Inspirations and Nature’s Place.
The USDA is reminding consumers to check their freezers as well as refrigerators as it continues to investigate.
The USDA said Hannaford kept limited records regarding the source of the ground beef and it is unable to determine the responsible supplier. The government agency said it will pursue rulemaking to address this problem in the future.
Salmonella is one of the most common bacterial foodborne illnesses. It can be life-threatening in individuals with weak immune systems, such as the elderly. Common symptoms include diarrhea, abdominal cramps, fever, headache, chills, nausea and vomiting. The food poisoning symptoms can start within 12 to 72 hours of food consumption and last up to seven days.
Preventing Salmonella
The USDA advises consumers to take special care in handling ground beef to avoid Salmonella.
Wash Your Hands. Consumers are urged to wash their hands with warm, soapy water for at least 20 seconds before and after handling food.
Separate. Keep raw meat, fish and poultry away from other foods that will not be cooked. Use separate cutting boards for different meats and egg products.
Cook Properly. Cook meat to safe internal temperatures. The safe internal temperature for beef and pork is 160 degrees Fahrenheit and 165 degrees for poultry. Use a food thermometer to check.
Refrigerate Immediately. Refrigerate raw meat and poultry within two hours of purchase or one hour if kept in temperatures of 90 degrees or greater.
Click here to read the full USDA notice on the Hannaford Ground Beef Recall.
Read More
Medical Device Recall for CooperVision Contact Lenses
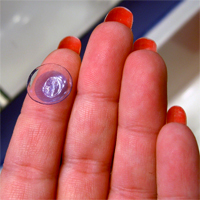 Consumers are advised to stop using a second type of CooperVision’s Avaira contact lenses due to a defect that may result in blurry vision and other eye injuries.
Consumers are advised to stop using a second type of CooperVision’s Avaira contact lenses due to a defect that may result in blurry vision and other eye injuries.
On Nov. 16, an unknown quantity of CooperVision Avaira (enfilcon A) Sphere contact lenses were recalled due to the unintended presence of a silicone oil residue.
CooperVision, a Fairport, NY-company, recalled the lenses in cooperation with the Food and Drug Administration (FDA), which states that the silicone oil residue can result in a wide range of symptoms, including eye discomfort, hazy and blurry vision and eye injuries requiring medical treatment. The FDA regulates contact lenses as a medical device.
The recalled lenses were manufactured from Feb. 1, 2011 to Aug. 24, 2011 and distributed from March 2, 2011 to Nov. 15, 2011.
The contact lenses were used to correct myopia and hyperopia and non-aphakic individuals with non-diseased eyes. The lenses may be worn by individuals who have astigmatism of 2.00 diopters or less that do not interfere with visual acuity.
Lens wearers have been instructed to stop using the lenses immediately and contact their eye care professional. They can visit the CooperVision recall website and enter the defective medical device package numbers to determine if their lenses have been recalled.
The medical device recall expanded another in August 2011 for limited lots of Avaira Toric contact lenses. CooperVision said the product defect on that line has been corrected through its quality system process.
Contact lens injuries are prevalent, due to product defect or improper use. They are most common among children and adolescents. In a study published in the July 2010 Pediatrics, FDA researchers reported 23 percent of total medical device injuries in 2004 and 2005 in the U.S. involved children and contact lenses. Children ages 11 and over were the most affected.
Common personal injuries include corneal contusions and abrasions, hemorrhage and conjunctivitis.
Click to read the FDA notice about the CooperVision recall.
Read More
Food Recall for Ocean Spray Dried Cranberries
 As the Christmas season begins, one Massachusetts company remains bogged down in Thanksgiving, after issuing a recall for its popular dried cranberry snacks.
As the Christmas season begins, one Massachusetts company remains bogged down in Thanksgiving, after issuing a recall for its popular dried cranberry snacks.
The day after Thanksgiving, Ocean Spray of Middleboro issued a recall for four package sizes of its Original Flavor Craisins Dried Cranberries product. The company voluntarily recalled the snack because of the possibility packages contained small hair-like metal fragments. The metal fragments resulted from an equipment malfunction on a production line, a company official told Vitals, an MSNBC.com blog.
Ocean Spray said it has received no consumer complaints or injury reports. Dried cranberry snacks are a growing product line for Ocean Spray. Last summer, a company official told the New England Business Bulletin that it was planning to expand its Middleboro facility and produce a third dried cranberry product.
The following Ocean Spray Original Flavor Craisins Dried Cranberries are part of this product recall:
- 5 oz Craisins® UPC: 00293-000
- 10 oz Craisins® UPC: 29456-000 and 29464-000
- 48 oz Craisins® UPC: 00678-318
- 10 lb bulk ingredient & foodservice UPC: 03477-000
Click here to see the full list with production dates for the recalled products.
Consumers who have purchased the recalled products are advised to discard them and preserve the UPC label and best by date. They should contact the Ocean Spray Consumer Hotline at 1-800-662-3263 for a coupon replacement.
Read More
Dangerous Toy Report Details Lead, Choking Hazards
 For a quarter century, the U.S. PIRG Education Fund (U.S. PIRG) has educated the public about toy safety for the holiday shopping season.
For a quarter century, the U.S. PIRG Education Fund (U.S. PIRG) has educated the public about toy safety for the holiday shopping season.
This year, U.S. PIRG’s “Trouble In Toyland: The 26th Annual Survey of Toy Safety,” warns consumers about over a dozen potentially dangerous toys, including those with dangerous levels of lead, phthalates and cadmium. Other warnings were issued for toys posing choking and strangulation hazards as well as those that create an excessively loud noise. The Boston product liability lawyers at Breakstone, White & Gluck urge you to glance at the report’s findings before you do your holiday shopping:
Toys With Dangerous Lead Limits. Lead has been banned in paint, children’s products and cookware in the United States since 1978, but it remains a problem in toys which are imported from other countries. Between October 2010 and November 2011, nearly 200,000 toys were recalled because of excessive lead limits, U.S. PIRG reported.
This year’s U.S. PIRG review found two toys on the market with lead levels which exceed the current 300 ppm standard, one toy that exceeded the prospective 100 ppm standard and four toys that exceeded the American Academy of Pediatrics recommendation of 40 ppm. This is the limit supported by U.S. PIRG.
Phthalates in Toys. Exposure to phthalates has been linked to have adverse health effects for children in the womb, including premature delivery and reproductive defects. U.S. PIRG identified two toys with limits exceeding the amount allowed by the Consumer Product Safety Improvement Act of 2008. The two toys include the Funny Glasses, manufactured by Joking Around, and the Sleep Mask, manufactured by Claire’s. Both exceed the 1000 ppm of banned phthalate standard.
Cadmium. Cadmium is a frequent additive in children’s jewelry. In response to a legal action in September, retailers agreed to sell only products that have less than 300 ppm cadmium. U.S. PIRG found no cases of noncompliance, but urged the Consumer Product Safety Commission to proceed with setting limits for cadmium in children and toy jewelry.
Choking Hazard. Choking on toys with small parts is the leading cause of toy-related deaths and injuries. Between 1990 and 2010, over 200 children in the United States died from choking.
Toy makers face complex regulations for testing and labeling their products. U.S. PIRG found several violations, including toys with no warning labels indicating they included small parts. The group called on the Consumer Product Safety Commission to take actions such as enlarging the small parts test tube size.
Loud Toys. U.S. PIRG reported several toys on the market exceed the ASTM 2003 standards for sound-producing toys, including Elmo’s World, Talking Cell Phone, manufactured by Fisher-Price; Victorious Stereo Headphones, manufactured by Nickelodeon; and Hotwheels, Super Stunt RAT BOMB, manufactured by Hotwheels.
Our recommendations:
- Shop wisely. If you have small children in the house, avoid toys for your older children which might represent choking hazards for your younger children.
- Avoid products which are obviously intended to generate loud noises or which may have headphones that cannot be turned down.
- Common sense says look for toys that have been on the market for more than a year because they are more likely to have been tested and proven safe.
- Buy toys that will promote physical activities to help your children stay strong and healthy.
To read the full U.S. PIRG “Trouble In Toyland: The 26th Annual Survey of Toy Safety,” click here.
Read More

