Posts Tagged ‘defective’
Defective Medical Devices Gain Criticism from Consumer Reports
 Consumer Reports is stepping into the debate about the medical device approval process, recommending that the Food and Drug Administration require more rigorous testing to prevent defective medical devices from going to market.
Consumer Reports is stepping into the debate about the medical device approval process, recommending that the Food and Drug Administration require more rigorous testing to prevent defective medical devices from going to market.
The magazine and its advocacy arm Consumers Union wants the FDA to require implants and other medical devices undergo testing to prove they are safe and effective. The FDA began classifying medical devices into three categories in 1976 and stated manufacturers would be required to show clinical data before approval of Class III products, the most at-risk category.
But the FDA routinely clears new medical devices under a process known as 510, in which manufacturers are required to bypass clinical testing if they can show a device is “substantially equivalent” to another device already on the market.
Consumers Reports is calling on Congress to require testing as part of the FDA’s approval process for medical devices. Next, it wants the practice of “grandfathering” high-risk implants stopped. Finally, the organizations seek an improvement to the system for notifying patients of medical device failures.
Currently, the system largely relies on physicians who are supposed to notify patients, but this is a problem when doctors stop practicing.
Without changes to the system, Consumer Reports said patients cannot properly protect themselves.
The magazine highlighted three types of defective medical devices which have caused injuries in recent years:
Surgical Mesh: This device was approved several years ago based on its relationship to a product used in the 1950s, even though the two products were inserted differently and treated different areas of the body. The FDA refused calls to recall surgical mesh, but in January ordered 33 companies to conduct the first-ever post-market safety studies of the product. The FDA is also considering reclassifying surgical mesh into a Class III category.
The Consumer Reports article shares the story of a 54-year-old woman who has undergone eight surgeries to correct her transvaginal mesh complications.
Artificial All-Metal Hips: DePuy Orthopedics recalled its ASR XL all-metal hip implant in 2010 after the FDA received about 400 complaints in two years from patients. The two metal parts were rubbing against each other, breaking and spreading metal particles into the blood stream. Injury reports about all-metal hip implants grew after that, with the FDA receiving more than 5,000 reports about hip implants in the first six months of 2011, according to a New York Times article. DePuy hip implants was estimated to account for 75 percent of those injury reports.
Related:
- Consumer Reports Investigates: Dangerous Medical Devices
- Protect Yourself Against Medical Device Injuries
Recalled Birth Control Pills: Lo/Ovral-28, Norgestrel and Ethinyl Estradiol
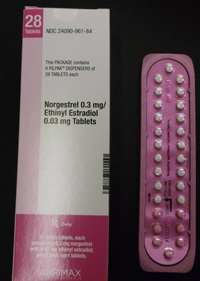 Pfizer Inc. has voluntarily recalled certain lots of birth control pills which may contain ingredient errors or out-of-sequence packaging which could have exposed women to a risk for unintended pregnancy.
Pfizer Inc. has voluntarily recalled certain lots of birth control pills which may contain ingredient errors or out-of-sequence packaging which could have exposed women to a risk for unintended pregnancy.
In January, Pfizer recalled 14 lots of Lo/Ovral-28 (norgestrel and ethinyl estradiol) Tablets and 14 lots of Norgestrel and Ethinyl Estradiol Tablets (generic) for customers in the U.S. market. The defective pills were distributed to warehouses, clinics and retail pharmacies nationwide.
An investigation by Pfizer found that some blister packs may contain an inexact count of inert or active ingredient tablets and that the tablets may be out of sequence. Pfizer recalled the tablets on January 31, 2012, with knowledge of the Food and Drug Administration (FDA). Pfizer said the error has been corrected.
The tablets were manufactured and packaged by Pfizer Inc., commercialized by Akrimax Rx Products and labeled under the Akrimax Pharmaceuticals brand. The medicine is packaged in blister packs of 21 tablets of active ingredients and seven tablets of inert ingredients. Click the link below for packaging numbers involved in the recall.
The product liability lawyers at Breakstone, White & Gluck are reviewing cases for women who have taken defective lots of these birth control pills and have experienced or are experiencing an unplanned pregnancy. Contact us today at 800-3791244 or 617-723-7676 or use our contact form. Read More
Product Recall: More than 10,000 Fuji Bicycles Recalled
 The U.S. Consumer Product Safety Commission announced the recall this week of more than 10,000 defective bicycles after reports the product’s frame was breaking.
The U.S. Consumer Product Safety Commission announced the recall this week of more than 10,000 defective bicycles after reports the product’s frame was breaking.
Fuji Saratoga Women’s Bicycles recalled about 10,500 bicycles sold nationwide from November 2007 through December 2011. The bicycles were recalled after the company became aware of 12 reports of bicycle frames breaking. There were two injuries reported, including a head laceration requiring 20 stitches.
The product’s defect is its frame was breaking in the center of the downtube during use, causing bicyclists to lose control and fall. Bicyclists are instructed to stop riding and seek a replacement bike frame.
About the recalled Fuji women’s cruisers bicycles:
- 2008 to 2010 models of Saratoga 1.0, Saratoga 2.0, Saratoga 3.0 and Saratoga 4.0. The model type will be printed on the bike.
- The bikes come in various colors.
- The bikes will have the words “Fuji” and “Saratoga” alone or “Saratoga” printed on the frame.
- Serial numbers beginning with ICFJ7, ICFJ8, ICFJ9, ICFJ10 and ICFJ11. The serial number is located on the bottom of the frame near the crank.
The defective products were imported by Advanced Sports, Inc. of Philadelphia and manufactured in China. They were sold at specialty bike shops.
Customers are instructed to obtain a free replacement frame. They can contact Advanced Sports Inc. toll-free at 888-286-6263 between 8 a.m. and 4:30 p.m. Monday through Friday or visit www.fujibikes.com. They can also return the bike to any authorized Fuji Bicycle dealer for the free part.
Click here for more information on this recall.
In a smaller recall, the Mountain Bicycle Handlebar Stem has been recalled in the U.S. and Canada. Some 213 units were recalled in the U.S. and 83 in Canada by the importer, Shimano American Corp. of Irvine, Calif. The defective bicycles were sold at REI stores nationwide from October 2009 to November 2010 for about $120.
The bicycles were recalled because the bolt holding the front plate of the stem to the stem body can be pulled out of the threads while the bike is being ridden, causing the rider to fall. There has been one report of a rider falling and sustaining torso and arm injuries. Click here for more information on this recall.
The Boston product liability lawyers at Breakstone, White & Gluck have over 80 years combined experience handling complex cases involving serious personal injuries, wrongful death and defective products. We have obtained clients compensation in cases involving defective motor vehicles, recalled medical devices and dangerous pharmaceuticals.
If you have been injured, it is important to learn your rights and how long you may have to file a claim. For a free legal consultation, contact us today at 800-379-1244 or 617-723-7676 or use our contact form.
DePuy Hip Implant Recall Leads Insurers to Seek Recovery
Medical insurance companies are alerting patients who have defective all-metal hip implants that they will seek to recover expenses from any settlement money which patients receive. Medicare is also expected to try and recover its costs.
Thousands of patients have been affected by recent recalls of all-metal hip implants and are expected to seek compensation from manufacturers. Although insurance companies, by statute or contract, often have the right to recover some of their costs, it is unusual for companies to be so assertive for medical device failures. The aggressive actions being taken by the insurers reflect their awareness of just how expensive these defective products are becoming.
In August 2010, DePuy recalled the ASR XL Acetabular System. The hip implant is defective because it causes friction between the metallic ball and socket components, which are implanted to replace the femur and acetabulum.
The recalled DePuy hip implants can wear down and produce a substantial amount of metallic particles in patients’ bloodstreams. Patients reported signs of metallosis, including pain, swelling, problems walking and rashes. Other serious problems may include bone fractures, joint inflammation, hip dislocation and damage to the tissue, hip implant loosening, nerves and muscles near the implant.
DePuy recalled the defective hip implants after the Food and Drug Administration (FDA) received about 400 complaints in two years from patients. That number has grown significantly following the recall. In just the first six months of 2011, the FDA received more than 5,000 reports about problems with all-metal hip implants, according to an analysis by The New York Times. DePuy hip implants accounted for 75 percent of the complaints.
It is still unclear how many patients have been affected by defective metal hip implants. The New York Times reports one estimate that 500,000 patients have received an all-metal replacement hip.
The newspaper reports another estimate that 250,000 hip replacements are performed in the United States each year. Until recently all-metal hip implants accounted for nearly one-third of these procedures.
As of October 2011, 3,500 patients filed lawsuits against DePuy in connection with last year’s recall. DePuy also faces over 560 lawsuits in connection with the Pinnacle, another defective all-metal hip implant.
More lawsuits are expected against DePuy and other manufacturers as patients start experiencing pain and requiring corrective surgery. This procedure can be painful, require substantial post-operation bed rest and treatment and cost hundreds of thousands of dollars.
Click here to read more about all-metal hip implants in the New York Times.
Read More
Infant’s Death Prompts Walmart To Remove Baby Formula
 Walmart pulled Enfamil baby formula from more than 3,000 stores this week after a newborn’s death in Missouri.
Walmart pulled Enfamil baby formula from more than 3,000 stores this week after a newborn’s death in Missouri.
Walmart voluntarily removed the cans of Enfamil Newborn from its store shelves on Monday night. The baby formula is under investigation by health officials after the 10-day-old baby boy’s death Sunday from Cronobacter, a bacteria linked to newborn illness and milk-based powder baby formula. The formula had been used by the baby’s family. A second infant was infected by the bacteria, but recovered.
The 12.5-ounce cans of Enfamil Newborn are marked with the lot number ZPIK7G. This includes at the chain’s 35 stores, 12 super centers and 2 Sam’s Club locations in Massachusetts.
The formula has been sent for testing to the U.S. Centers for Disease Control and Prevention (CDC) and the Food and Drug Administration (FDA). In the meantime, the Missouri Department of Heath and Senior Services is urging consumers who purchased the formula to return it to the store or discard it.
The manufacturer Mead Johnson said that the batch of infant formula used by the child’s family tested negative for Cronobacter when it was produced and packaged.
Mead Johnson and Walmart representatives say the companies are working with authorities. Neither of the companies has implemented a formal product recall. The government has not requested one.
Click here to read a recent news article about this product liability case.
Read More
Toyota Recalls 550,000 Toyotas and Lexus Models
 A defective crankshaft pulley is driving a new Toyota recall that will affect thousands of car owners. The Nov. 8 recall of 550,000 vehicles includes 420,000 cars in the United States. Toyota has now issued recalls for more than 13 million vehicles nationwide since September 2009. More vehicles have been recalled in other countries.
A defective crankshaft pulley is driving a new Toyota recall that will affect thousands of car owners. The Nov. 8 recall of 550,000 vehicles includes 420,000 cars in the United States. Toyota has now issued recalls for more than 13 million vehicles nationwide since September 2009. More vehicles have been recalled in other countries.
Toyota recalled the vehicles due to a crankshaft pulley defect that may cause steering problems. The vehicles have V6 engines and the U.S. models include 283,200 Toyota brand cars like Camry and Highlander vehicles and 137,000 Lexus vehicles.
Toyota said no injuries have been reported. The car manufacturer said the crankshaft pulley may have an inadequate amount of adhesive agent between the outer ring and the inner ring. This can cause the crankshaft pulley to become misaligned with the inner ring, possibly causing a noise or warning signal to light. When this happens, the belt for the power steering pump can detach from the pulley, making it hard for drivers to steer.
Toyota recalled 8 million vehicles between Nov. 2009 and the first quarter of 2010, most for defective pedals. In April 2010, the United States government fined the world’s largest automaker a record $16 million for its delayed response in notifying the National Highway Traffic Safety Administration regarding the defects.
Recalls have continued through 2011, including last February’s recall of 2.17 million vehicles to repair mechanical defects that could cause the cars to accelerate out of control.
The Nov. 8 recall involves these defective motor vehicles: the 2004 and 2005 Camry, Highlander, Sienna and Solara; the 2004 Avalon; and the 2006 Highlander HV. The affected Lexus models are the 2004 and 2005 ES 330 and RX 330 and the 2006 RX 400h.
In January, Toyota will mail car owners a notification to make an appointment to have their vehicle inspected. Toyota will fix any vehicle in need of repair at no cost to consumers.
Read More
Honda and Ford Recall More than 2 Million Vehicles
 Two major manufacturers have issued car recalls impacting more than three million drivers.
Two major manufacturers have issued car recalls impacting more than three million drivers.
Ford last week announced the recall of more than 1.22 million pickup trucks because of a corrosion problem that can result in a gas tank falling off and catching on fire. The recalled trucks include the popular F-150.
The Ford recall affects older trucks sold between 1997 and 2004 in Canada, Washington D.C. and 21 cold-weather states where salt is used on the roads to prevent icing in the winter. Massachusetts is among those states.
The Michigan car manufacturer plans to notify affected owners in September and will repair the trucks for free. The models include: Ford F-150 (1997-2003), the 2004 F-150 Heritage, the F-250 (1997-1999) and the Lincoln Blackwood (2002-2003).
Ford said it has received eight reports of tanks falling, resulting in three injuries.
This is the second recall action involving Ford trucks in four months. In April, Ford expanded a recall of F-150 pickups to about 1.2 million trucks. In that case, the problem was the front-seat airbags could deploy without a motor vehicle crash.
Also last week, Honda Motor Co. announced the recall of more than 2.49 million cars, SUVs and minivans with defective transmission software. The defect can affect a car’s transmission if the software cannot keep up with movements, such as a vehicle trying to emerge from the snow or a driver moving between gears.
The Honda recall includes 1.5 million vehicles in the U.S., about 760,000 in China and 135,000 in Canada.
Globally, the recall affects four-cylinder Accord sedans (2005 – 2010). In the U.S. and Canada, the car recall also includes the CR-V crossover (2007 to 2010) and the small Element SUV from (2005 to 2008).
The company said no injuries or wrongful deaths have been reported due to the defect.
Honda will begin contacting U.S. consumers at the end of August about updating the software. The update will be free.
Read More
Product Recall: Bunk Beds from Big Lots
 A popular bunk bed set is being recalled after a three-year-old boy became entrapped in the frame and died due to asphyxiation.
A popular bunk bed set is being recalled after a three-year-old boy became entrapped in the frame and died due to asphyxiation.
Big Lots of Columbus, Ohio is recalling 30,000 metal futon bunk beds sold at its stores nationwide from January 2009 through April 2010 for about $200.
The recalled beds have a convertible futon bed on the bottom with a metal ladder leading up to a twin-sized bed. The Consumer Product Safety Commission (CPSC) warns parents that children behind the futon or in the ladder area can become entrapped when the futon and its metal frame are lowered to the flat position.
In March 2010, a young boy from Burlington, Iowa suffered a wrongful death after his head and neck got caught in the bunk bed. The child was unable to breathe and died at the hospital due to compression asphyxiation.
An additional hazard is the space between the last rung on the defective bunk bed’s ladder and the futon mattress is too small, posing a head and neck entrapment hazard.
The Big Lots recall involves metal futon beds with model number BFB1008 located on a label on the upper bunk. The defective beds were sold unassembled and were manufactured in China.
Consumers should immediately stop using the defective bunk beds. They can contact Big Lots for a free repair kit containing a new ladder and other parts.
Consumers can contact Big Lots at (866) 244-5687 during business hours Monday through Friday or visit the retailer’s website at www.biglots.com.
Big Lots has stores throughout Massachusetts, including in Lynn, West Bridgewater, Franklin, Milford, Methuen, Attleboro, Raynham Center, Worcester, Fitchburg, Seekonk, Swansea, Dudley, Gardner, Springfield and Northampton.
Read More
Time to Think Back to School Safety
Heading back to school is always a big event, no matter how old a student is. Students look forward to meeting new teachers, starting new classes and being reunited with friends.
But all this activity brings safety concerns. Yet if parents, teachers and students recognize the risks and work together, the Back-to-School season can be an enriching time. Here are some tips to keep your children safe:
Playgrounds. Each year, more than 200,000 children are treated in U.S. emergency rooms for falls on the playground. The goal is to implement preventative measures in your playground and make it as safe as possible if falls do occur.
Start by inspecting playground equipment for any defective or broken parts.There should be a 12-inch depth of wood chips, mulch or sand. Mats should be made of safety-tested rubber or fiber material to prevent head injury if a child falls.
Drawstrings on Jackets and Sweatshirts. Many pieces of fall clothing come with drawstrings. Most people think nothing of these until a child endangers himself or a classmate, often unknowingly.
Prevent a dangerous situation where a child gets strangled. Remove drawstrings on hoods. Cut drawstrings from the waist or bottom of jackets, coats and sweatshirts to three inches.
Loops on Window Blind Cords. Visit your child’s classroom to ensure it’s a safe environment. Look at the windows to see if they have blinds with a long cord. If there are blinds with cords, this is a safety hazard. A child could strangle himself when the teacher’s not looking or swallow the plastic piece at the end of the cord.
Bikes. Many students ride their bicycles to school. It’s important for drivers to watch out for them, but parents also need to educate students on how to avoid bike accidents. The first rules is bike helmets. Massachusetts has a mandatory bike law for minors under 16. Beyond the law, bike helmets prevent and reduce head injuries should your child take a fall.
To learn more about school safety, visit the Consumer Product Safety Commission web page, “American Goes Back to School Program.”
Read More

